ORGANIC
CHEMISTRY (HYDROCARBON)
Organic chemistry is the study of
compounds of carbon. Nearly
all organic compounds also contain hydrogen; most also contain oxygen,
nitrogen, or other elements .
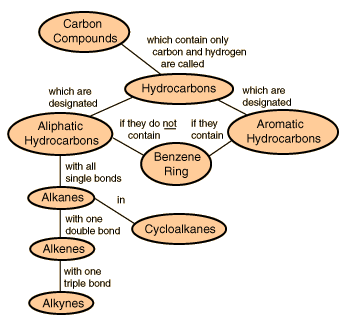
Hydrocarbons are the simplest organic compounds
. Containing only carbon and hydrogen, they can be straight-chain,
branched chain, or cyclic molecules. Carbon tends to form four bonds in a
tetrahedral geometry. Hydrocarbon derivatives are formed when there is a substitution of a functional group at one or more of these positions.
The majority of hydrocarbons found naturally occur in crude oil, where decomposed organic matter provides an abundance of carbon and hydrogen which, when bonded, can catenate to form seemingly limitless chains.
Hydrocarbon frameworks
Chains
The simplest
class of hydrocarbon frameworks contains just chains of atoms.
Names
for carbon chains
Functional groups
1.
Alkanes contain
no functional groups
The
alkanes are the simplest class of organic molecules because they contain no
functional groups.
They
are extremely unreactive, and therefore rather boring as far as the organic
chemist is concerned.
However,
their unreactivity can be a bonus, and alkanes such as pentane and hexane are
often used as
solvents, especially for purification of organic compounds.
2.
Alkenes
(sometimes called olefins) contain C=C double bonds
It
may seem strange to classify a type of bond as a functional group, but you will
see later that C=C
double
bonds impart reactivity to an organic molecule just as functional groups
consisting of, say,
oxygen
or nitrogen atoms do. Some of the compounds produced by plants and used by
perfumers are alkenes
3.
Alkynes contain
CºC triple bonds
Just
like C=C double bonds, CºC triple bonds
have a
special
type of reactivity associated with them, so it’s
useful
to call a CºC triple bond a functional group.
4. Alcohols (R–OH) contain a hydroxyl (OH) group
Molecules
containing hydroxyl groups are often soluble in water, and living things often
attach sugar groups, containing hydroxyl groups, to otherwise insoluble organic
compounds to keep them in solution in the cell.
5. Ethers (R1–O–R2) contain an alkoxy group (–OR)
The
name ether refers
to any compound that has two alkyl groups linked through an oxygen atom. ‘Ether’
is also used as an everyday name for diethyl ether, Et2O.
6. Amines (R–NH2) contain the amino (NH2) group
We
met the amino group when we were discussing the amino acids: we mentioned that
it was this group that gave these compounds their basic properties. Amines
often have powerful fishy smells: the
smell
of putrescine is particularly foul. It is formed as meat decays. Many
neurologically active compounds
are
also amines: amphetamine is a notorious stimulant.
7.
Nitro compounds
(R–NO2)
contain the nitro group (NO2)
Several
nitro groups in one molecule can make it quite unstable and even explosive.
Three nitro
groups
give the most famous explosive of all, TNT (trinitrotoluene), its kick.
8.
Alkyl halides (fluorides
R–F, chlorides R–Cl, bromides R–Br, or iodides R–I)
contain the fluoro, chloro, bromo, or iodo groups
These
three functional groups have similar properties—though alkyl iodides are the
most reactive
and
alkyl fluorides the least. PVC (polyvinyl chloride) is one of the most widely
used polymers—it
has
a chloro group on every other carbon atom along a linear hydrocarbon framework.
Methyl
iodide
(MeI), on the other hand, is a dangerous carcinogen, since it reacts with DNA
and can cause
mutations
in the genetic code
9.
Aldehydes
(R–CHO) and ketones (R1–CO–R2) contain the carbonyl group C=O
Aldehydes
can be formed by oxidizing alcohols—in fact the liver detoxifies ethanol in the
bloodstream
by
oxidizing it first to acetaldehyde (ethanal, CH3CHO). Acetaldehyde in the blood is the cause of hangovers.
1 Carboxylic acids (R–CO2H) contain the carboxyl group CO2H
As
their name implies, compounds containing the carboxylic acid (CO2H) group can react with bases,
losing
a proton to form carboxylate salts. Edible carboxylic acids have sharp flavours
and several are
found
in fruits—citric, malic, and tartaric acids are found in lemons, apples, and
grapes, respectively.
1 Esters (R1–CO2R2)
contain a carboxyl group with an extra alkyl group (CO2R)
more volatile esters, have pleasant, fruity
more volatile esters, have pleasant, fruity
smells
and flavours. These three are components of
the
flavours of bananas, rum, and apples:
1 Amides (R–CONH2, R1–CONHR2, or R1CONR2R3)
Proteins
are amides: they are formed when the carboxylic acid group of one amino acid
condenses
with
the amino group of another to form an amide linkage (also known as a peptide
bond).
1 Nitriles or cyanides (R–CN) contain the cyano group –CºN
Nitrile
groups can be introduced into molecules by reacting potassium cyanide with
alkyl halides.
The
organic nitrile group has quite different properties associated with lethal
inorganic cyanide:
Laetrile
1 Acyl chlorides (acid chlorides)(R–COCl)
Acyl
chlorides are reactive compounds used to make esters and amides. They are
derivatives of carboxylic
acids
with the –OH replaced by –Cl, and are too reactive to be found in nature.
1 Acetals
Acetals
are compounds with two single bonded oxygen atoms attached to the same carbon
atom.
Many
sugars are acetals, as is laetrile which you have just met.
Covalent Bonding in
Organic Compounds
A carbon can
have four single bonds, two double
bonds, a double and two single bonds, or
a triple and a single bond; all total
four bonds. These bonds can be
represented by electron dot or line
bond formulas.
Electron
Configuration of Carbon
Bonding in carbon involves the
promotion of a 2s electron to an empty 2p
orbital thus creating four unpaired
electrons, one in the 2s and one in each of
the three 2p orbitals. This allows
carbon to be tetravalent.
Shapes of Organic
Molecules
The shapes of organic molecules
are predicted using the following
principle: atoms and non-bonding
electron pairs attached to a common
central atom are arranged as far apart
in space as possible. If there are four
surrounding groups, the shape is tetrahedral;
with three, the groups protrude
to the corners of a triangle
(trigonal); and with two, the region is linear.
Bonding in Organic
Compounds – A Summary
A carbon with four bonded groups is
tetrahedral, sp3-hybridized, and has
109.5O bond angles. A carbon with three is trigonal,
sp2-hybridized, and has
120O bond angles. A carbon with two bonded groups
is linear, sp-hybridized,
and has 180O bond angles.
A single bond is a sigma bond; a
double bond is composed of one sigma
bond and one pi-bond; a triple bond is
one sigma and two pi-bonds.
Triple bonds are stronger than double
bonds and double bonds are
stronger than single bonds. The
opposite order describes relative bond
lengths.
Tidak ada komentar:
Posting Komentar